Mitochondrial function decline - one of the aging reasons
|
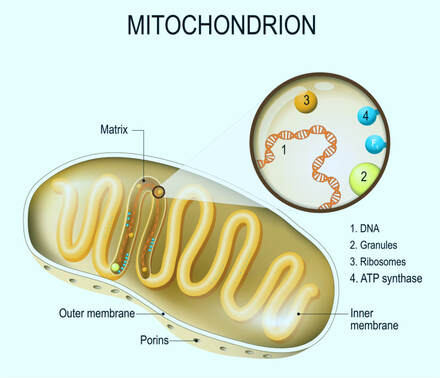
What are mitochondria?
A mitochondrion, plural - mitochondria, is an organelle within our cells (the exception is mature red blood cells that have no mitochondria). Mitochondria play multiple roles, the most well known of which is to absorb nutrients from a cell, break them down, and convert them into energy (called ATP, adenosine triphosphate) that the cell can use. As an interesting side note, mitochondria have their own DNA (MtDNA), and it is only passed to a child from Mother, never from Father. Thus, if you do your genetic testing, just like X chromosome can be used to discover a strictly paternal lineage, MtDNA shows a strictly maternal lineage. |